The atomic model proposed by J.J. Thomson revolutionized the field of atomic physics in the late 19th century. This model introduced the concept of electrons and laid the groundwork for future developments in atomic theory. In this article, we will delve into the intricacies of Thomson's atomic model, exploring its historical context, key features, and the subsequent advancements that followed. By the end of this comprehensive guide, you will have a clear understanding of how Thomson's work shaped our current knowledge of atomic structure.
The significance of J.J. Thomson's atomic model cannot be overstated. Before his contributions, the understanding of atoms was limited, and many questions regarding their structure remained unanswered. Thomson's experiments with cathode rays led to groundbreaking discoveries, establishing a new framework for the study of atomic particles. This article aims to provide a detailed exploration of Thomson's atomic model, its implications, and its relevance in today's scientific landscape.
As we embark on this journey through the world of atomic theory, we will examine not only the scientific principles behind Thomson's model but also the broader impact it had on the field of physics. Through a series of well-structured sections, we will ensure that you gain a thorough understanding of the topic. Let's dive in!
Table of Contents
1. The Historical Context of J.J. Thomson's Work
J.J. Thomson, a British physicist, made significant contributions to the field of atomic physics during a time when the understanding of matter was still in its infancy. Born in 1856, Thomson's academic journey began at Trinity College, Cambridge, where he studied mathematics and physics. His fascination with electricity and magnetism led him to investigate cathode rays, which ultimately paved the way for his groundbreaking atomic model.
1.1 The Scientific Landscape of the Late 19th Century
During the late 1800s, scientists were grappling with various theories about the nature of matter. The prevailing belief was that atoms were indivisible, a notion that dated back to ancient Greek philosophers. However, as experimental techniques advanced, researchers began to uncover evidence suggesting that atoms were composed of smaller particles. Thomson's work emerged during this scientific revolution, challenging the long-held views of atomic structure.
2. Key Experiments Leading to the Atomic Model
Thomson's atomic model was primarily derived from his experiments with cathode rays. In 1897, he conducted a series of groundbreaking experiments that revealed the existence of electrons, the negatively charged particles within atoms. By using a cathode ray tube, Thomson demonstrated that these rays were composed of particles much smaller than atoms.
2.1 The Cathode Ray Experiment
In his cathode ray experiment, Thomson applied an electric field to a gas-filled tube, which caused the cathode rays to bend towards the positive electrode. This observation indicated that the rays were composed of negatively charged particles. Through meticulous measurements, he calculated the charge-to-mass ratio of these particles, ultimately leading to the identification of electrons as fundamental components of atoms.
3. The Structure of Thomson's Atomic Model
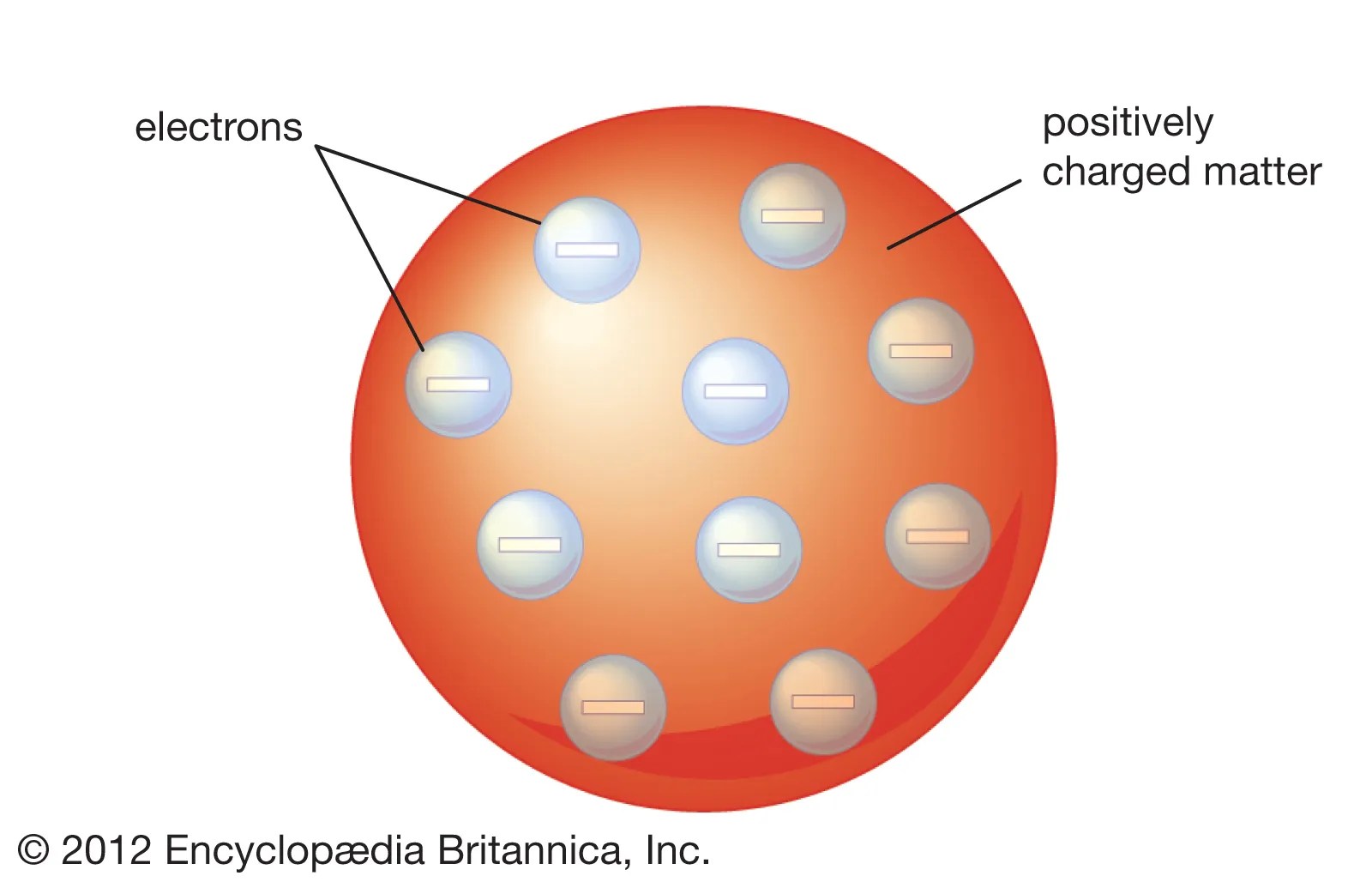
Thomson's atomic model, often referred to as the "plum pudding model," proposed that atoms were composed of a positively charged "soup" with negatively charged electrons embedded within it. This model represented a significant departure from the previous belief in indivisible atoms and introduced the concept of subatomic particles.
3.1 Key Features of the Plum Pudding Model
- Positively Charged Matrix: Thomson envisioned the atom as a uniform distribution of positive charge, providing stability to the structure.
- Embedded Electrons: Electrons were thought to be scattered throughout the positive matrix, similar to plums in a pudding.
- Neutral Overall Charge: The model maintained that the total charge of the atom remained neutral due to the balance between positive and negative charges.
4. Implications of the Thomson Atomic Model
Thomson's atomic model had profound implications for the field of physics and chemistry. It not only provided a framework for understanding atomic structure but also influenced subsequent research and theories. The introduction of electrons as fundamental particles opened up new avenues for exploration in atomic physics and chemistry.
4.1 Impact on Chemical Reactions
The recognition of electrons as key players in atomic structure led to a deeper understanding of chemical reactions. Thomson's model suggested that electrons were involved in the formation of chemical bonds, reshaping the way scientists perceived the interactions between atoms.
5. Limitations of Thomson's Model
While Thomson's atomic model was groundbreaking, it was not without its limitations. As experimental techniques advanced, it became clear that the plum pudding model could not adequately explain certain phenomena, such as the results of Rutherford's gold foil experiment.
5.1 Inability to Explain Atomic Stability
Thomson's model struggled to account for the stability of atoms, particularly the behavior of electrons in relation to the positively charged matrix. This limitation prompted further investigation and ultimately led to the development of new atomic models.
6. The Transition to Rutherford's Model
In 1911, Ernest Rutherford conducted the famous gold foil experiment, which challenged the validity of Thomson's plum pudding model. Rutherford's findings revealed that atoms consisted of a dense, positively charged nucleus surrounded by orbiting electrons. This discovery marked a significant shift in the understanding of atomic structure and prompted the development of the nuclear model of the atom.
6.1 The Evolution of Atomic Theory
The transition from Thomson's model to Rutherford's model represented a pivotal moment in the history of atomic theory. Rutherford's work laid the foundation for future advancements, including Niels Bohr's planetary model and later developments in quantum mechanics.
7. Modern Perspectives on Thomson's Contributions
Despite the limitations of his model, J.J. Thomson's contributions to atomic physics remain invaluable. His pioneering work on cathode rays and the discovery of electrons fundamentally changed the landscape of science and paved the way for future discoveries in the realm of atomic structure.
7.1 Recognition and Legacy
Thomson's legacy extends beyond his atomic model. He received the Nobel Prize in Physics in 1906 for his investigations of the electrical conductivity of gases. His impact on the scientific community continues to resonate, influencing generations of physicists and chemists alike.
8. Conclusion and Future Directions
In conclusion, J.J. Thomson's atomic model was a milestone in the evolution of atomic theory. By introducing the concept of electrons and challenging the prevailing notions of indivisible atoms, Thomson laid the groundwork for future advancements in atomic science. While his model had limitations, its significance in shaping the understanding of atomic structure cannot be understated.
As we move forward in the field of atomic physics, it is essential to appreciate the foundational contributions made by Thomson and recognize how they paved the way for modern scientific inquiry. We encourage you to engage with this topic further by leaving comments, sharing this article, or exploring additional resources on atomic theory.
Thank you for taking the time to delve into the fascinating world of atomic models. We hope to see you back on our site for more in-depth explorations of scientific concepts!
ncG1vNJzZmivp6x7rLHLpbCmp5%2Bnsm%2BvzqZmpJ2cocZur86lpqurX5bBsLnInGSmp5SauW62yWaroaedqLyvesetpKU%3D